- Home
- Types & Treatments
- Immunotherapy
- Immunotherapy FAQ
Immunotherapy
Immunotherapy FAQ
Immunotherapy and cellular therapeutics represent the future of cancer care. These biological therapies involve manipulating the body’s cells to reactivate and strengthen their abilities to attack cancer cells. As a National Cancer Institute-designated comprehensive cancer center – 1 of just 54 in the nation – we are among the leaders in advancing and delivering revolutionary precision cancer therapies.
Immunotherapy is a precision cancer treatment and is considered the future of cancer treatment by the National Cancer Institute. Researchers and cancer experts at The University of Kansas Cancer Center are at the forefront of research to advance the use of immunotherapy treatments against cancers. Other, more traditional, types of cancer treatment include surgery, chemotherapy and radiation.
Speaker 1: From the research bench to the patient's bedside, this is Bench to Bedside with your host, Vice Chancellor and Director of the University of Kansas Cancer Center, Dr. Roy Jensen.
Darren McLaughlin is thankful to be alive. He celebrated anniversaries and family birthdays and moved into a new home that he and his wife built. Events he didn't think he would be around to see two and a half years ago when he has done diagnosed with Burkitt's lymphoma. Fortunately, Darren was referred to the University of Kansas Cancer Center where he received CAR T-cell therapy to treat his rare and fast growing B-cell non-Hodgkin's lymphoma. The CAR T-cell therapy Darren received, Yescarta, was first approved by the US food and drug administration in 2017 as a third line treatment for adults with large B-cell lymphoma who had already undergone two rounds of treatment that had failed.
Our cancer center was a leading study site for the clinical research trial that determined Yescarta was superior to the long standing second line standard of care, which is a stem cell transplant following high dose chemotherapy to kill the lymphoma. The results from the global clinical trial have literally changed the standard of care for treating large B-cell lymphoma.
Roy Jensen: Hi, I'm Dr. Roy Jensen, Vice Chancellor and Director of the KU Cancer Center. And with me to talk about this exciting advancement is Dr. Joel McGuirk, medical director of the blood and marrow transplant program at the university of Kansas Cancer Center, and his patient Darren McLaughlin, who received CAR T-cell therapy for large B-cell lymphoma.
First of all, I want to thank both of you for joining us for today's episode of Bench to Bedside, and I'm going to start with you, Dr. McGuirk. Tell us about the ZUMA-7 clinical trial and the significance of these early results.
Joseph McGuirk: Absolutely. Critically important trial. It's driven a paradigm shift and change in the way. We're looking at the treatment of patients with large cell lymphoma that doesn't go into remission with their initial chemotherapy, or does go into remission and subsequently relapses. The former was the case for Mr. McLaughlin. Resistant to chemotherapy upfront, just not responding. Couldn't get him into complete remission. This therapy, CAR T-cell therapy, has been approved as you know, in the third line. That is in patients who have failed a stem cell transplant previously, or who are not responding to chemotherapy. And then another type of chemotherapy is given, they still aren't responding. So what's called a third line. Once it demonstrated success in that clinical setting, it raised a question of, should we move this earlier in our armamentarium against these cancers, against these very aggressive blood cancers before patients are too far down the line and less likely possibly to respond.
And so this study was a multicenter, multinational trial that put head-to-head CAR T-cell therapy in a patient who relapses or who won't go into remission in the first place up against high dose chemotherapy and stem cell transplant, the standard of care until now. And that perspective randomized trial, the event free survival, which means patients either progressed and required further therapy, or they died from some cause, oftentimes if they died from relapse progressive disease. That was the population and showed a highly statistically significant improvement in event free survival, 0.0001. And that's been announced nationally.
These data will be presented in the plenary session in the American Society of Hematology meeting coming up soon and hope will soon be submitted for publication. University of Kansas Cancer Center was a lead enrolling site in this multi-center, multi-national trial. And we're thankful to patients like Darren who submitted themselves to this trial so that we could move the ball down the field. And I believe, end up with the paradigm shift such that CAR T-cell therapy is now the second line standard of care therapy for these patients.
Roy Jensen: That's incredibly exciting. So, Dr. McGuirk, if I understand correctly, we are the only cancer center in the region to offer all five FDA approved CAR T-cell therapies. Tell us exactly what is CAR T-cell therapy and what was our cancer center's specific role in the ZUMA-7 clinical trial?
Joseph McGuirk: Absolutely. So CAR T-cell therapy, in the form of the FDA approved indications, five different indications. And as you mentioned, we were among the first 14 centers in the country to have the first two indications and quickly a frontline center for the subsequent three indications. And it continues to expand in its application.
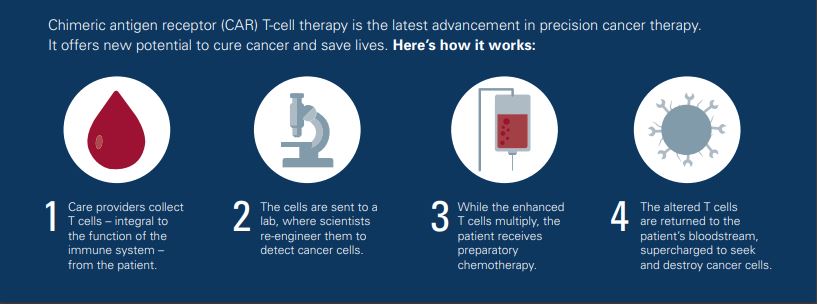
CAR T-cells involve leveraging our better understanding of how the immune system works to control cancer and what goes wrong when it doesn't control cancer. Leveraging that knowledge and taking patients own T-cells out of their blood, to the laboratory, genetically engineering them such that they'll recognize the cancer again, attached to the cancer cells, punch a hole in them, release molecules that go in and chop up their DNA, their insides, and kill the cancer cell.
And as you mentioned, we have been on the forefront of this. So we were the lead enrolling site, and the first to enroll in a multi-center, multi-national trial. Juliet published in the new England journal of medicine of CAR T-cell therapy in patients who were not responding to chemotherapy in the frontline or subsequently relapsing, including after stem cell transplant. That gave rise to FDA approval for the construct KYMRIAH and then subsequent constructs is as well.
And so our center, the reason we have been one of the first centers to employ and be approved to provide these five currently commercially available therapies is because we developed a reputation for intellectual rigor, clinical trials, office and support staff in the cancer center that allowed us to very safely and robustly enroll in these clinical trials and provide data that were very clearly had a great deal of integrity. And when you develop that reputation, you have the infrastructure to support these trials, people come looking for you, and that's basically what's happened here.
Roy Jensen: So this new therapeutic approach is approved for patients who relapse from the first round of therapy from large B-cell lymphoma. How often does that happen? And what does these results mean for them?
Joseph McGuirk: That's a really important question. In diffuse large cell lymphoma, about 25,000 patients are diagnosed on a yearly basis out of those 25,000 patients, about 50 to 60% will be cured with their frontline chemotherapy and not require such treatment. However, that leaves us with about 40 to 50% of patients who don't. So eight to 12,000 patients who don't.
The standard of care until now has been such patients receive another cycle of chemotherapy to see if their tumor responds. If it responds to the chemotherapy, then they go on and receive intensive chemotherapy in an autologous stem cell transplant. And so this calls that into question. The current commercial indications for CAR T-cell therapy are for the third line. So patients have to have failed two attempts, two different types of therapies. This challenges that and says, "Perhaps we should move it up earlier. And when they fail their first therapy, employ CAR T-cell therapy, and perhaps the patients will do better." And what we see so far in the ZUMA-7 trial is that they do much better indeed.
Roy Jensen: So Darren, thank you so much for joining us today. Could you share your story with us and the events that led up to your diagnosis with Burkitt's lymphoma?
Darren McLaughlin: Sure. Well, I appreciate the opportunity to do so. In about October of 2018, I had started suffering from some lower back pain. This was, I thought, was due to the fact I was moving our household, truckload by truckload. And I just thought I was suffering from back pains from that. But it got progressively worse to the point that in January of 2019, I was taken to the emergency room because I was having trouble breathing and the pain was just becoming so bad. And it was there I was diagnosed with stage four Burkitt's lymphoma. And I was told it was a very aggressive form of lymphoma. And they started chemotherapy right away.
And the initial chemotherapy had some positive result. But after the second round of chemotherapy, the tumor was continuing to grow. And it was at that point, I was told that there wasn't a lot more that that current hospital staff could do. They just didn't have any other treatment options open. But the doctor said that I have made a referral to Dr. McGuirk over at KU because they have treatment options, one of those being Car T available for you to try if you're accepted. And fortunately, Dr. McGuirk did accept me, thought my case was viable for CAR T treatment. And I did receive CAR T treatment around May of 2019.
Roy Jensen: And so what was your experience like with CAR T as opposed to chemotherapy?
Darren McLaughlin: The CAR T treatment did not bother me at all. Very little long term side effects. I had some of the predictable side effects that they told me that I would possibly have, but I found it to be I could very much handle what I went through and compared it to the chemotherapies. I think it was less stressful on my body than the chemotherapies were. I had had several rounds by that time. And so, I was still, I was pretty weak by the time I got the CAR T treatment, but I think I went through the CAR T treatment pretty well, actually.
Roy Jensen: That's great. So, Dr. McGuirk, what do you think the results of this study mean in the big picture for the field of cancer treatment?
Joseph McGuirk: Let me qualify something that both you and Darren have mentioned, Dr. Jensen and Darren, with all due respect. The outside institution diagnosed Darren with Burkitt's lymphoma. However, our [inaudible 00:13:14], who focused just on these blood cancers, highly specialized and unique, determined that it was not indeed Burkitt's lymphoma. That it was a high grade B-cell lymphoma. Burkitt's lymphoma patients were not included in this trial. The high grade B-cell lymphoma population was. So just for clarification on that issue.
With regard to what does it mean? Well, we're a small community CAR T researchers around the nation and we all know each other. And University of Kansas is in a large consortium of centers that provide CAR T-cell therapy. And once the data became available, several weeks ago that we had this significant event-free survival difference, favoring CAR T-cell therapy, people started sending emails to each other. And I sent a two word email from University of Kansas Cancer Center and said, "Paradigm shift." And that got a lot of follow on that, yes, indeed, our colleagues around the nation agreed, this is a paradigm shift for our patients. It's going to change the way that we treat patients in the second line. So it's a really big deal. And I think that that's clearly why it made the plenary session for American Society of Hematology meeting.
Roy Jensen: Absolutely. Well, it's an important point you bring up about correct diagnoses, and that's not that unusual at an NCI designated cancer center to have that be a critical feature of selecting a patient's care, because he would not have been eligible if that diagnosis had been left in place.
Joseph McGuirk: That's right.
Roy Jensen: So obviously, we can't do a Bench to Bedside without talking about COVID. And could you tell us about how COVID-19 has impacted the care of BMT and CAR T patients at the University of Kansas Cancer Center.
Joseph McGuirk: Yes, absolutely. It's been extremely impactful. Patients who undergo stem cell transplantation and patients who receive CAR T-cell therapy are very immunodeficient. Their immune system is not set up at all to handle simple cold viruses that we have to deal with in the community, no less than SARS-COV-2, the virus that causes COVID-19.
And so we reported, we published last month in the Journal of Transplant and Cell Therapy, our results, and in our transplant patients who developed COVID-19, 28% of patients died and that's been duplicated in Western Europe and throughout the United States. So our patients are highly vulnerable. A more recent publication of pulling together data from many centers, including our own, showed that our CAR T patients also are greatly vulnerable. And why is that? Well, to make antibodies to fight this, for example, when we have a vaccination, you have to have cells called B-cells, and B cells are the cells that became cancerous, for example, in Darren.
So our therapy, CAR T-cell therapy, is aimed at B-cells, his cancer cells, but collateral damage is that we knock out his normal B-cells for many months after the therapy. So he can't make antibodies, even when we vaccinate him. Now our standard of care, NCCN guidelines nationally, we vaccinate our CAR T patients and our transplant patients three months after the procedure. However, knowing the B-cell population, and we study this carefully in our center as you know, after transplant and CAR T-cell therapy to see, are their B-cells recovered? Are they making antibody yet? Most are not by three months. So they receive that first and second vaccination, but that third vaccination is critical to this population, as well.
Even with all that having been said, many of our transplant and CAR T patients are still very vulnerable to this virus. It could become very sick, hospitalized and even die from this after having been vaccinated. Of the patients who are dying from COVID that have been vaccinated, it's these types of patients that are the most vulnerable. And so social distancing, wearing a mask in any public space, and making sure all household contacts and caregivers are vaccinated is critically important to the wellbeing and safety of our CAR T and stem cell transplant patients.
Roy Jensen: So, Darren, I know you developed COVID after you were vaccinated and you have a job where you're in front of the public all the time. So could you tell us about that and what precautions you and your family take with you being a lymphoma survivor?
Darren McLaughlin: Absolutely. Well, I am immune deficient. I still am immune deficient, being treated for that. And so, COVID was definitely one of those things that was topping our list, but I have to be careful about all different types of diseases and things. So we monitor my health and the activities I do. I do wear masks, make sure that I clean my with a hand sanitizer on a regular basis when I'm out doing things. My family is very cautious. They've been vaccinated. And yeah, I did come down with COVID about 40 days after I received my full round of vaccinations. Luckily, thankfully, with the very minor case of COVID, had very few symptoms, but I do believe the vaccination really helped me get through that disease with very few symptoms and issues.
But I'm still, even though I've had COVID, even though I've been vaccinated, I'm going get my booster here pretty soon, and I'm still careful. And I will be careful the rest of my life, even after COVID dies down. I've still got to be careful with the flu and common cold and all that stuff because my body just doesn't react to those things the same way and I am more susceptible. So it's constant diligent on my part and my family's part, but I'm just thankful for the fact that I still get to be here to be vigilant and it's a small price to pay for the alternative.
Roy Jensen: Absolutely. So any parting thoughts, Dr. McGuirk?
Joseph McGuirk: Yes, absolutely. This is a revolution that we're in the midst of in cancer therapeutics, as you know, better than anyone, Dr. Jensen. Immunotherapy is a critically important part of that. Using these cells to attack cancer will only expand including into solid tumors. We have a number of clinical trials at our cancer center focused on solid tumors now, and not just the blood cancers that we've been treating with CAR T-cell therapies. A lot more to come. We have a lot of work to do. These therapies do have toxicities. Not everyone benefits, not everybody goes into complete remission and is potentially cured. And until that's the case, we have a lot of work to do. We do that in the context of clinical trials. And so they are obligated in a cancer center like ours to have a broad spectrum of clinical trials to avail our patients of. Very much committed to that.
Roy Jensen: Darren, any parting thoughts?
Darren McLaughlin: Yeah. I'm just very thankful to Dr. McGuirk and the rest of the doctors and nurses that were instrumental in my treatment. My family and I never pass up an opportunity to do things like this today to espouse the great work that KU, Dr. McGuirk, and all the other people that I've had the benefit of contacting through the course of this disease. It's truly great to have such an institution here in this area, and very thankful that they were there to help me. [inaudible 00:21:28]
Roy Jensen: Well, thank you, Darren, for being on today's show and sharing your story. And thank you, Dr. McGuirk. That's it for today. To learn more about this issue, please visit KUCancerCenter.org. That's it for today. Join us next week at 10:00 AM for Bench to Bedside. Thanks for watching.
Subscribe to our Bench to Bedside podcast. Now everywhere podcasts are available.
Request your appointment today.
To make an appointment at The University of Kansas Cancer Center, call 913-588-1227.