October 07, 2019
It’s not an exaggeration when I say we’re in the middle of a revolution in treating blood cancers, such as leukemia, lymphoma and myeloma.
Doctors have a vast understanding of how these cancers work, what causes them and the effects they have on patients. This comes from decades of basic and clinical research into these cancers and how the immune system responds to them.
And now that knowledge is leading to better, smarter and more effective methods of fighting blood cancers. One advanced treatment method involves training a patient’s immune system to better fight cancer cells.
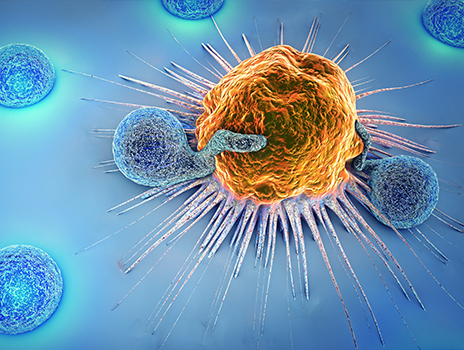
We can now take special cells from a patient’s immune system called T cells and arm them with weapons to hunt for and destroy blood cancer cells. These reengineered T cells are known as CAR T cells.
What are T cells?
There are many kinds of T cells. All of them are part of the immune system, where they play important roles in recognizing and fighting diseases. Some T cells search for invading cells and identify them. They do this by binding with the invading cells and signaling the rest of the immune system to find and destroy the cells to which they bind.
But cancer cells are crafty. They trick the immune system and continue to grow and develop inside the body without being destroyed. For example, a blood cancer cell can create “keys” on its surface to turn off a T cell that tries to bind to it, much like turning off the ignition of a car. The T cell becomes inactive, and the cancer can continue to reproduce and spread.
Another way blood cancer cells outwit the immune system is by creating decoys. This causes the immune system to attack the decoys instead of the true cancer cells. Our research allows us to arm the immune system with smarter, more powerful tools in the form of CAR T cells.
What are CAR T cells?
CAR T cells, or chimeric antigen receptor T cells, are extraordinary weapons in the fight against blood cancers. These are versions of T cells doctors extract from a patient’s blood and change so they’re better able to fight cancer cells and then reintroduce into the patient’s body.
Doctors can train T cells to recognize a specific protein that is on the surface of cancer cells and locate those cancerous cells in the body. My team has helped research this process.
In 2016 and 2017, we worked with patients who had diffuse large B-cell lymphoma. This cancer affects B cells, which are another type of immune-system cell. Diffuse large B-cell lymphoma is the most common aggressive form of non-Hodgkin lymphoma.
Most cases of diffuse large B-cell lymphoma are treated with chemotherapy. Each of these patients had received multiple chemotherapy treatments and a bone marrow transplant from my team, and nothing had worked. The patients were expected to survive only another 6-12 weeks.
We drew T cells from these patients’ blood then sent them to a lab outside our facility where a protein was added to shield the T cells from the cancer cells’ ability to deactivate them. T cells were reengineered to recognize a protein on the outside of the B cells called CD19. This is possible by removing the inner parts of a virus, leaving only its shell. Then genes are inserted into the virus shell to teach the T cells to recognize the CD19 protein.
Next, technicians at the lab outside our facility infected the patient’s T cells with this virus. The special genes inserted into the virus became part of the T cells’ makeup. These re-engineered T cells began to grow special receptors on their surfaces called chimeric antigen receptors, or CARs.
After billions of these CAR T cells were grown in the lab for each patient, they were sent back to us and we put them back into the patients’ bloodstreams. The shielded T cells were able to locate and bind themselves to the cancer cells, where they signaled the immune system to send additional support. These immune cells punched holes in the cancer cells and injected toxins that tore the cancer cells apart. Once the cancer cells were destroyed, the remains were removed from the body with other waste products.
The results were amazing. About half of the patients we treated with CAR T cells showed no further signs of cancer. We call that complete remission.
We offer a wide range of clinical trials like these affiliated with the National Cancer Institute that aren’t available elsewhere in the region.
The revolution in cancer care continues
Doctors are now using CAR T cells to treat a number of blood cancers besides diffuse large B-cell lymphoma, including:
- Acute lymphoblastic leukemia
- Acute myelogenous leukemia
- Chronic lymphocytic leukemia
- Multiple myeloma
And we’re starting to see this type of treatment for other cancers as well, such as breast cancer, lung cancer and colon cancer. CAR T cells have the potential to be the next evolution in cancer treatment.
We’re only beginning to understand how CAR T cells and other methods of engineering T cells will allow us to provide better, faster and smarter cancer treatments. We're excited to see where further research takes us and the improvements in patients’ lives that will be possible with this technology.

Cancer-free after a clinical trial
A grandmother diagnosed with acute lymphocytic leukemia is cancer-free thanks to an immunotherapy clinical trial.